Whether you’re part of a large, well-established company with an existing Quality Management System, or involved in a start-up that’s looking to implement its first QMS, a tailored, fit-for-purpose QMS solution is essential to achieving and maintaining compliance as a certified legal manufacturer. In our topical article below, our Head of Quality Dr Dirk Hüber shares advice on how to choose the right QMS for your company, covering why an effective QMS is important, the factors to consider that’ll influence your QMS, and how to implement a QMS that meets your business needs.
Why an effective QMS is so important
For start-ups taking their initial steps onto the market, establishing a reliable QMS system that facilitates compliance with ISO 13485 is a fundamental requirement for achieving certification as a legal manufacturer. Whilst the implementation of a QMS might be triggered by the nearing of the registration deadline (with focus more on product development than on formal processes), it’s important to apply due diligence to the QMS set-up to ensure it’s comprehensive, lean, and scalable in the long term.
For larger companies with long time established and certified Quality Management Systems, optimising ongoing effectiveness is often the prompt to perform a major QMS change. Usually, the QMS has grown in complexity over time and as a result become inefficient, and most likely to some extent ineffective.
Not having an optimal QMS for your company can be costly. Inefficiencies can create unnecessary work that lacks value, and ineffectiveness can induce issues, non-conformances, and audit findings that may require considerable resources to fix.
On the other hand, implementing your first QMS, or making a major change to your existing QMS also comes with a price tag. However, the benefits of implementing an optimal QMS far outweigh the cost. For start-ups, a solid QMS foundation will support the enhancement of the core business. And for larger companies, if you calculate the costs spent in the last 2-3 years on issue resolution (e.g., on NCs, CAPAs, SCARs and audit observations), and compare this with the cost of a major overhaul of your QMS, you’ll find that the efficiencies gained with a QMS improvement quickly pay off.
Factors that influence your QMS
To choose the right QMS for your individual business, you first need to understand which factors influence a QMS, and the consequences of such influences.
Applicable regulations
Depending on the nature of your products or services, the regulations applicable to your company influence the processes within your QMS, because they establish the requirements your QMS must meet.
Most of these requirements are universal – they are independent of the nature of your product/s. A few requirements are product-specific, for example, requirements for sterilization processes only apply to products with sterile components.
The regulations do not require a specific structure for a QMS. Nevertheless, certain structural elements have become common, especially for processes not related to product realisation. Similarly, processes meeting universal requirements naturally always look alike, even across industries: for example, a CAPA is a CAPA, regardless of whether you’re a MedTech or IVD firm or a pharmaceutical company.
In essence, the high level structure of a QMS could be the same for a MedTech, an IVD, or even a Pharma company, although many processes will differ in content, some considerably.
Product class
The classification of medical and IVD devices is risk based: for medical devices according to the risk the device poses to patients and users, and for IVD devices according to the risk the disease that shall be diagnosed, monitored, etc., poses to the patients and public. So, one may question, whether this means that if a device is of a lower risk class, a “simpler” QMS will suffice?
The answer is “no”. You need the same processes, whatever the classification might be. What differs with the classification are the requirements for the devices themselves. Within some processes, you have to do less for lower class devices, e.g., in product registration, or post market. But you still need the respective processes in your QMS.
Company size
In most companies with a long established QMS, the number of QMS documents correlates with the number of employees, whereby the correlation factor is usually between 10 and 100. That’s to say, for each employee there are about 10 to 100 QMS documents! Usually, there are more QMS documents per employee for larger companies because many authors of QMS documents tend to describe the tasks of their functional silo, instead of processes. And as the company grows, the organisation can become more fractioned, and more functional descriptions are needed.
A side-effect of such a function-oriented QMS is that interfaces between the functions tend to become neglected, and instructions for different functions may become inconsistent, since the focus is mostly only on the individual function. This leads to inefficiencies and, if the interfaces are broken, non-conformances.
Age of the QMS
The instructions within a QMS become more and more complex over time. Such complexity may reach a level where employees are no longer able to fully understand and/or follow the instructions, and as a result non-conformances become inevitable. This happens because whilst the authors want to mitigate non-conformances, ironically, they actually create more non-conformances due to their approach.
When a non-conformance is detected, the “easiest” approach is often to blame the process for being insufficient, and then add instructions to the process for the specific situation in which the non-conformance occurred. Over time, the QMS will accumulate more and more of these special instructions, making the QMS documents increasingly complex. Of course, this will not reduce the number of non-conformances that happen, since one cannot catch every potential event.
With some of the main factors that influence a QMS now understood, let’s take a look at how you can achieve an improved QMS, maintain the improved state over time, and prevent the system’s dissolution.
Implementing a QMS that meets your business needs
To achieve an effective QMS, your QMS needs a clear and easy structure, process orientation including process ownership, QMS oversight and a quality and compliance culture. These attributes are elaborated below.
Structure
The easiest way to structure a QMS is to implement a two-tier structure following ISO 13485. On the upper tier, chapters 4-8 are represented as overarching topics, into which the various processes are then assigned. Figure 1 below shows how such a QMS structure may look:
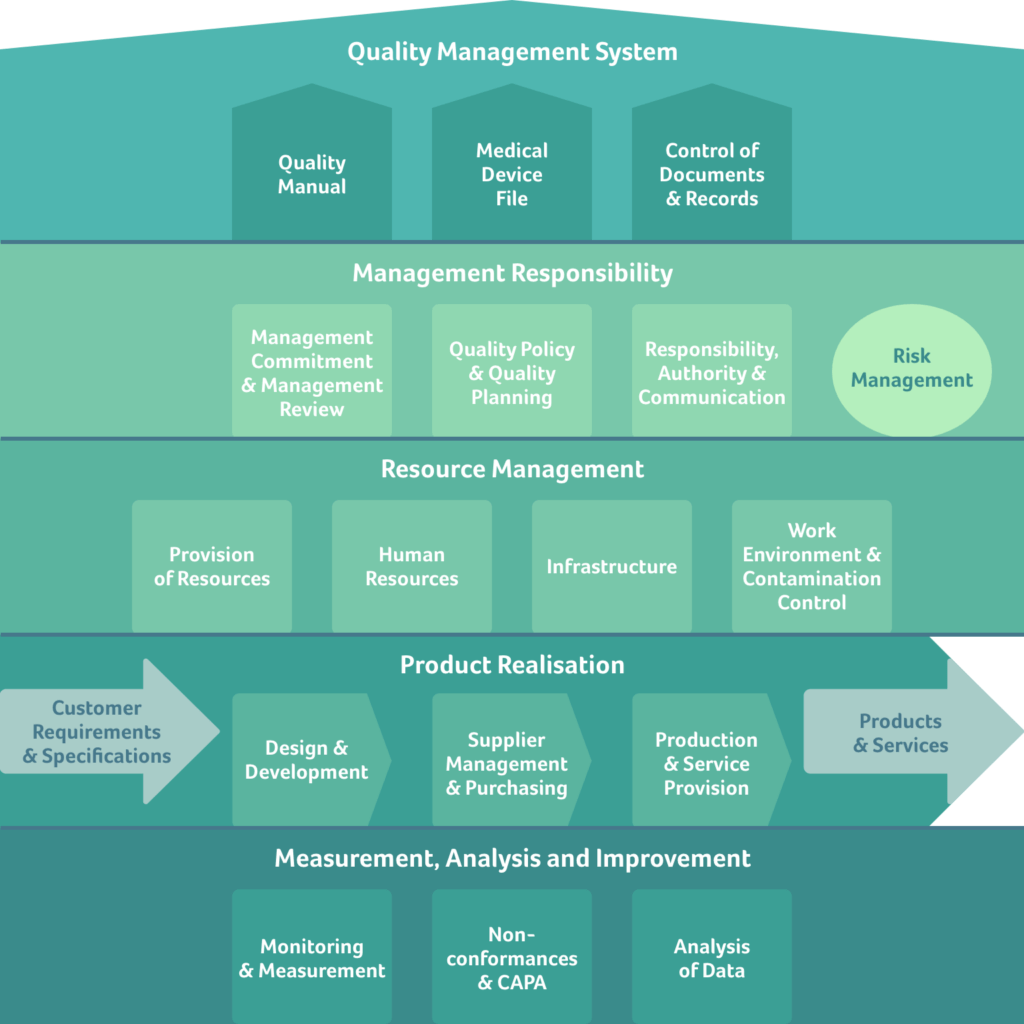
Figure 1: An example QMS structure based on ISO 13485. In addition to the requirements from ISO 13485 a risk management process is included (mint green circle), which follows the requirements in ISO 14971. The 17 processes shown in this example are structured in 5 groups, according to chapters 4-8 of ISO 13485, with risk management assigned to management responsibility. Note that MDR or IVDR requirements, respectively, must also be included within the applicable processes.
The number of SOPs necessary to describe a process depends to some extent on the complexity of the organisation and its products. However, you should be careful to limit the number of SOPs to an essential minimum to maximise efficiency.
Process orientation including process ownership
Process orientation must be built into each layer of your QMS. This is easiest done with process flows: each SOP contains a process flow showing the steps within the SOP, also showing interfaces to other SOPs within the same process and to other processes.
For the processes, a process flow would show how the SOPs that constitute this process, are connected to each other, and the interfaces to other processes. On the highest level, the QMS as a whole would have a process flow (shown in the Quality Manual) that shows how the layers of the QMS (e.g., the 5 levels in Figure 1) are connected to each other.
For a small QMS, this can be handled fairly easily with, for example, Visio process flows in the respective QMS documents. For a more complex QMS, a process management tool might be advisable.
Another important aspect of process orientation is process ownership. A process should be owned by one of the main stakeholders in the process (and not simply by the Quality department). The process owner is then accountable for involving other stakeholders in developing and maintaining the process. Thus, all stakeholders are accountable to contribute to the process description (including forms and templates), and they are accountable for doing this with the process as a whole in mind, not just their own needs.
This way, the QMS will become owned and lived by the organisation, and not, as sometimes seen, perceived as something forced onto the organisation by the Quality department.
QMS oversight
When building and maintaining your QMS, it’s important to entrust the oversight to someone with a deep process understanding in general, but who also has some knowledge on all processes that are part of the QMS.
The person accountable for the QMS must be able to understand the needs of the organisation and pragmatically balance these needs against regulatory requirements. This is the only way to achieve and maintain an efficient and effective QMS with consistent process interfaces.
Experience shows that a mainly formal oversight will soon lead to increased complexity within the QMS with subsequent decreased efficiency. It’s important to remember that the processes of the QMS are there to serve your organisation – your organisation is not there to serve the QMS and its rules.
Quality and compliance culture
The most important ingredient for an effective and efficient QMS is the quality and compliance culture within your organisation. If – in the extreme – the prevailing attitude within the organisation is “I do only what is prescribed”, and the prescribed requirements are always questioned, the QMS will soon become complex, difficult to use, and inconsistent, since the usual reaction to such an attitude is to try and capture every eventuality within the QMS. This is a safe recipe for failure.
Alternatively, if your organisation has a good understanding what quality and compliance means, and how it helps the organisation and each person to perform better, the QMS can be kept lean and straightforward.
Here’s how you can achieve this ideal state:
- Have top management act as role models throughout their communication, actions, and decisions regarding quality and compliance topics.
- Ensure the QMS team within your organisation acts not only as a controlling institution, but also as a service provider and adviser to all other functions. For example – should an approach proposed by another function not be suitable, the Quality department should propose an alternative solution rather than just a refusal to approve the approach.
If your organisation achieves the above, your QMS will add value by supporting people in their tasks.
QMgeniuS | A tailored QMS solution for start-ups
When it comes to structure and simplicity, here at Congenius we offer a standard QMS intended for small start-up companies. QMgeniuS is a complete QMS for MedTech and IVD companies.
A lean, compliant, and ready-to-use solution, QMgeniuS provides QMS essentials and state-of-the-art processes. Designed so that it may be handled by even the smallest companies, QMgeniuS can be implemented within 5-6 months for a transparent, reasonable price.
Certain processes within QMgeniuS, or elements therein, are also suitable for larger companies such as KMUs looking to simplify and streamline processes to maximise efficiency and maintain compliance.
QMgeniuS incorporates 40 years of experience in the development and implementation of successfully certified Quality Management Systems in companies of different sizes, with varying maturity and product portfolios.
If QMgeniuS sounds like a solution that could help your business, please do get in touch to request a demo and start the conversation.

