Last year in our topical article How to optimize your supplier management, we examined the key issues, challenges, and opportunities regarding supplier management – highlighting the importance of establishing clear communication of requirements and deliverables, considering technical, financial, and legal aspects and their possible interrelationships.
Building on this important concept of clarity in communications, this topical article brought to you by our Operations team, provides a deep dive into standardized manufacturing guidelines to be produced by legal manufacturers of medical devices, which facilitate the smooth design transfer of key components, expectations, and requirements to their suppliers.
Understanding the needs of all stakeholders
Depending on their role, the different stakeholders involved in the design transfer process have varied perspectives and needs, and face varying challenges:
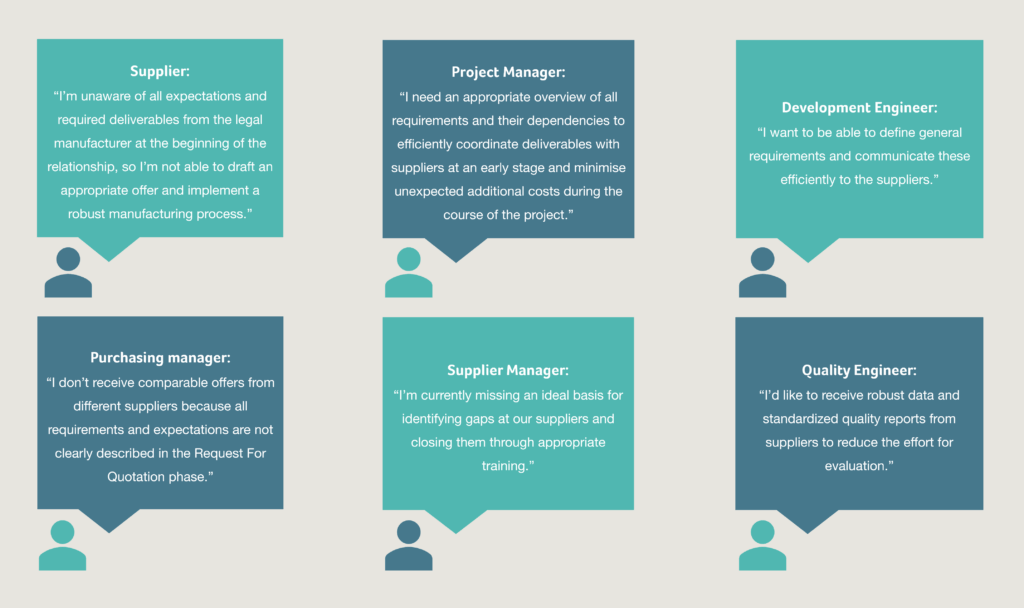
To summarise the collective considerations in the image above:
Technical drawings and design specifications (product design) are not always enough to transfer key information to suppliers. To ensure a stable and high quality manufacturing output, process requirements are essential.
To ensure supply chain resilience, second sources and interchangeable manufacturing assets are required.
Missing clarification and standardization can lead to increased supervision efforts with suppliers to guarantee alignment.
Quality levels can often differ from supplier to supplier, so it’s important to avoid complacency and assumption.
Producing clear manufacturing guidelines to address identified requirements
To overcome these challenges and considerations, legal manufacturers should aim to produce standardized manufacturing guidelines (ideally in a digital format) that cover the aspects below:
Enable ease of use
The digital platform used for the guidelines must be easy to share with various suppliers, and intuitive to use in terms of searching for, finding, and understanding the required topic. Effort to update the platform also needs to be kept to a minimum to avoid unnecessary costs and project delays.
Balance essential requirements with room to innovate
It’s crucial to include the correct level of detail in the guidelines to ensure that concepts and quotations are comparable. Bear in mind though, that suppliers may often bring additional perspective, experience, and expertise to the table – so balancing what’s absolutely necessary whilst allowing room for innovation or optimization will facilitate the most effective project outcomes.
Avoid overruling the supplier QMS
Whilst the guidelines require the adherence of suppliers, they are not intended to overrule the supplier’s Quality Management System (QMS), so approaching any sticking points with pragmatism and a collaborative mindset is advised to help overcome any challenges.
And when the time comes to implement the guidelines…
Personal interaction during kick-off, implementation, and supplier training will help to create rapport, and should encourage collaboration and accountability from the get-go. Furthermore, pro-active technical discussions at the beginning of the project, prior to implementation, can help to avoid late-stage problems that may require costly corrections.
The benefits of effective supplier guidelines
By following these general principles during the creation of their supplier guidelines, legal manufacturers can benefit from:
- The avoidance of interruptions to the supply of high runner / import products
- A significant reduction of effort during the implementation phase, for example, with reduced quality reviews, and clear Measurement System Analysis (MSA) requirements
- A reduction of lead times from development to production, including a reduction in Request for Quotation (RFQ) lead time by as much as 40%
- A greater awareness amongst suppliers regarding the need for legal manufacturers’ involvement in the design of the manufacturing process to ensure product quality
The industry more holistically also stands to benefit from the improved communication between legal manufacturers and suppliers:
Suppliers welcome standardization and clear means of communication that help to achieve a consistent quality standard across various suppliers and at multiple production sites (including second source), which helps to counteract quality challenges that can often occur as a result of decentralized manufacturing. For example, standardization and interchangeable tools between suppliers, e.g., in the injection moulding industry, have long been desired – and having this included as a requirement from the legal manufacturer helps to fulfil that need. More generally, guard rails and requirements help to prevent incorrect assumptions and lead to more efficient cooperation between the supplier and the legal manufacturer.
Standardization also aids continuous optimization. Standardized guidelines drive legal manufacturers to review, align, and optimize their internal processes. And more broadly, when all stakeholders work towards the same standardized goals, lessons learned and proven manufacturing concepts regarding tools, equipment, order processing, inspections etc… can be shared between manufacturing sites, suppliers, and legal manufacturers alike so that the industry can collectively improve.
In practice: Creating a supplier handbook
So, we have some guiding principles, but what are the practicalities involved in creating supplier guidelines? The successful implementation of a supplier handbook requires meticulous planning, collaboration, and continuous improvement. In our free factsheet available to download below, we outline a strategic approach and some tips for those developing a supplier handbook for the first time.
Can’t see the factsheet? Simply log in, or subscribe to our Knowledge update for full access to all content from our MedTech experts.
For more about our Operations services more generally, see here.


