A decentralized clinical trial (DCT) is a trial where subject recruitment, delivery and administration of study medication and collection of the trial’s outcomes data all occur without any face-to-face interaction between the trial participant and the study investigator. During COVID, remote solutions for clinical trials became more commonplace due to the ongoing risks, regulations and restrictions posed by the pandemic. With some sense of status quo now returning following a successful vaccination program, our Head of Clinical, Dr Barbara Widmann, reflects on whether decentralized methods will become the predominant approach for clinical trials moving forward.
The idea of a DCT is not a new concept. In 2011 Pfizer conducted the first completely decentralized randomised control trial in the pharma industry – the REMOTE trial. This completely web-based trial evaluated the efficacy and safety of an antimuscarinic in overactive bladder treatment, replicating previous on-site trials with only one study site managing the whole study.
Web-based tools were established for all steps of the trial from recruitment via screen to electronic informed consent, with e-diaries provided to the subjects for data entry, local lab testing performed near the participants’ homes, as well as the direct shipping of medication to the subjects. While the results of the trial were in line with previous findings and interest of potential participants was high (with over 5,000 initial registrations on the trial website), the web-based multi-step enrolment and consenting process proved too complicated. Resulting, only a few of the participants could be randomised, leading to an overall drop-out rate of 85%.
Despite substantial progress in digitalisation and improved scope for electronic data capture, both of which effectively facilitate remote trial methods, “traditional” trials have remained standard practice over the last decade.
In February 2020 however, COVID-19 disturbed the norm. When the pandemic hit, it disrupted many ongoing clinical trials, with subjects and clinical monitors either refusing to visit clinics due to fear of infection, or being restricted by site closures, quarantine, and travel limitations.
Sponsors were therefore forced to react quickly, by collaborating with clinics to find suitable remote solutions that ensured patient safety and limited the loss of important clinical data for the affected trials. The incorporation of remote methods such as remote recruiting, phone follow-up visits and data capture made it possible to convert clinical studies that were originally set up in a traditional, centralised format into at least partially decentralized trials, allowing the continuation of clinical investigations despite the challenges presented by a global pandemic.
These methods were supported by the authorities, with FDA and EMA both producing guidance documents on the conduct of clinical trials during the COVID-19 pandemic. These useful resources for those considering the implementation of remote practices when running a clinical trial are available here (FDA) and here (EMA).
But with life now returning to some sense of normality, what does the future hold for DCTs? Let’s take a look at the advantages and challenges of DCTs to help identify whether their adoption will change our post-pandemic clinical landscape for the better.
What are the main advantages of decentralized trials?
- Potential for faster recruitment by increasing patient access to clinical trials by removing the obstacles caused by having a specific trial location.
- Increased patient access to clinical trials may also lead to more diverse study populations which is especially important for the collection of real-world evidence.
- Likewise, there is potential to reduce dropout rates if the cost and time burden for subjects participating in a study is reduced.
- Streamlining trial conduct by reducing the number of trial sites involved, interacting with fewer ethical committees and switching to remote monitoring may decrease the overall costs of the trial significantly.
What are the main challenges of decentralized trials?
- Feasibility strongly depends on the treatment area to be studied. For example, DCTs might be less suitable for studies that require close patient monitoring in an early development phase, trials that assess an implantable medical device, or for studies with complex protocols.
- There is potential for bias in recruitment, especially if digital tools are being used for (as an example) an elderly subject group who have neither access to, nor an understanding of how to use the tool.
- Traditional endpoints might not be suitable for DCTs if they depend on assessment of lab values or other parameters that require a hospital environment for assessment.
- Reliable cybersecurity / data privacy systems are essential and might be more challenging to implement if patient data is being stored within a purely digital trial environment.
How do you find the “best” solution for both the patient and manufacturer?
While the concept of DCTs sounds promising, they are certainly not suitable to replace each and every type of clinical trial. If you would like to consider a DCT for your next study, you might find that running the study completely web-based proves even more complicated than a conventional on-site trial.
Therefore, you must first determine whether the medical device and therapeutic area are suited for a DCT at all by asking:
- Who is the intended user?
- What are the intended normal conditions of use?
- What study endpoints do you want to assess?
- Is it possible to collect the required data using remote tools?
You might consider involving the subject’s local General Practitioner or telemedicine provider for follow-ups in case you cannot fully rely on patient reported outcomes. In parallel this will decrease the burden on study participants and ultimately increase subject retention.
Furthermore, you will need to assess whether you have access to the right online tools to conduct the study as needed and to ensure data privacy – i.e. what solution will you use for subject screening and documentation of the informed consent process? The FDA has produced a guidance document for the use of electronic informed consent that gives valuable insight into viable options.
In addition, you need to consider the logistics of how to provide study subjects with the study medication or device and how to ensure they have the right training.
Assessing the need to have a 24/7 study hotline in place to monitor patient safety is also a consideration, and overall, you might want to engage your responsible ethical committee early on to get their opinion on your plans to run a DCT.
A hybrid approach to encourage recruitment, retention and diversity
When examining conventional centralised clinical trials in more depth, it is evident that many trials are already using some decentralized components such as telephone follow-ups in their current protocol. And for many areas of clinical research, this kind of hybrid approach, that incorporates remote components as far as possible in an otherwise traditional clinical trial may well present the best option to profit from the benefits of both formats.
For example, if you found yourself running a trial for an implantable medical device:
- Patient recruitment could take place at the clinic prior to the actual implantation.
- Follow-ups could be performed via telephone or internet to collect adverse event data and patient reported outcomes.
- Trial endpoints could be tailored to avoid the need for lab values or clinical tests.
- Mobile research staff or subjects’ local GPs could be utilised to realise remote data collection that does not require subjects to come back to a particular study site.
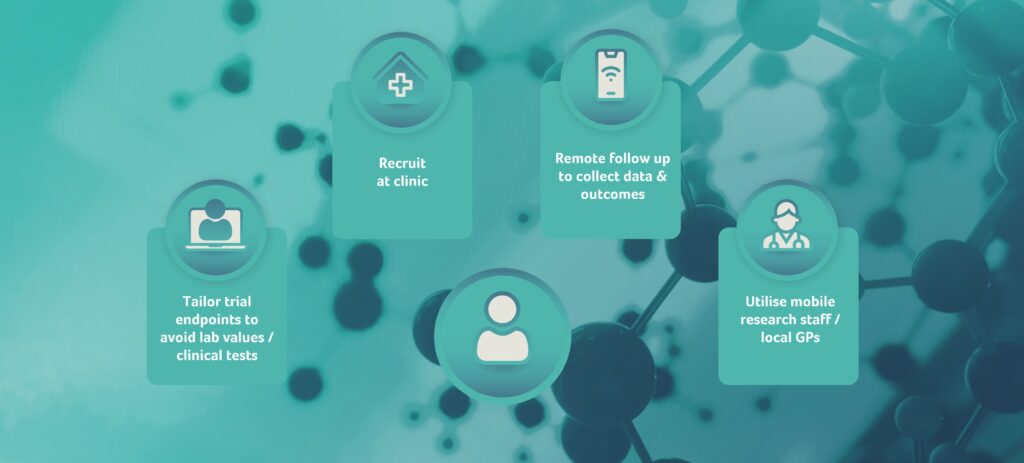
It will however, always be important to establish good contact between the study investigators and the participants to ensure safety oversight for all trial subjects.
One of the forces striving for increased quality and efficiency in clinical trials by developing current practices, is the Clinical Trials Transformation Initiative (CTTI). Founded in 2007, the CTTI is made up of over 80 industry organisations and agencies including the FDA. As part of their Mobile Clinical Trials Program, they recently released a white paper which provides valuable guidance on practical and regulatory considerations for planning and conducting a DCT.
Looking ahead, the prospect of adopting a decentralized approach for clinical trials is both feasible, and very worthwhile to explore. With digitalisation providing an increasing number of effective tools with which to conduct a clinical trial remotely, paired with the recent learnings collected during the COVID-19 pandemic, it has become clear that decentralising clinical trials at least on a partial basis, can save time and cost, whilst improving ease of recruitment, subject diversity and participant retention.
If you have a clinical trial related challenge, our Clinical team at Congenius are ready and happy to help. Simply get in touch to find out how we could support you.
Additional References
- Decentralized Clinical Trials: The Future of Medical Product Development? Van Norman GA, JACC Basic Transl Sci. 2021 Apr; 6(4): 384–387
- Decentralized Trials in the Age of Real-World Evidence and Inclusivity in Clinical Investigations. Khozin et al CLINICAL PHARMACOLOGY & THERAPEUTICS, VOLUME 106 NUMBER 1, JULY 2019
- The Clinical Trials Transformation Initiative (CTTI). Ann Ist Super Sanità 2011 | Vol. 47, No. 1: 14-18

