The Corporate Sustainability Due Diligence Directive (CSDDD), formally known as Directive (EU) 2024/1760, is a significant milestone in the European Union’s efforts to promote sustainable and responsible business practices. Adopted on 13 June 2024, and entering into force on 25 July 2024, the directive requires companies to analyse and monitor their business operations and supply chains for their impacts on human rights and the environment. This comprehensive initiative aligns with the EU’s objective of strengthening social responsibility and sustainable economic practices.
Following on from our 5-minute guide that summarised the EU Green Deal and its key directives, and our article on the CSRD, our topical article below explores the second of the three key directives underpinning the EU Green Deal that are most relevant to MedTech companies.
Keep reading as our Founder Jörg Dogwiler and Head of Regulatory Bruno Gretler explore the CSDDD’s requirements, its impact on the medical device industry, and the opportunities it presents for Swiss medical device manufacturers.
What’s the objective & scope of the CSDDD?
The Corporate Sustainability Due Diligence Directive (CSDDD) seeks to minimise human rights and environmental risks, holding companies accountable for addressing these risks in their global supply chains. The directive applies to:
- EU-based companies with more than 1,000 employees and global revenue exceeding €450 million
- Non-EU companies generating more than €450 million in revenue within the EU
Within this broad scope, the directive targets large corporations with significant influence over global supply chains. The aim is to combat systemic issues such as child labour, exploitation, and environmental degradation.
What are the core requirements under the CSDDD?
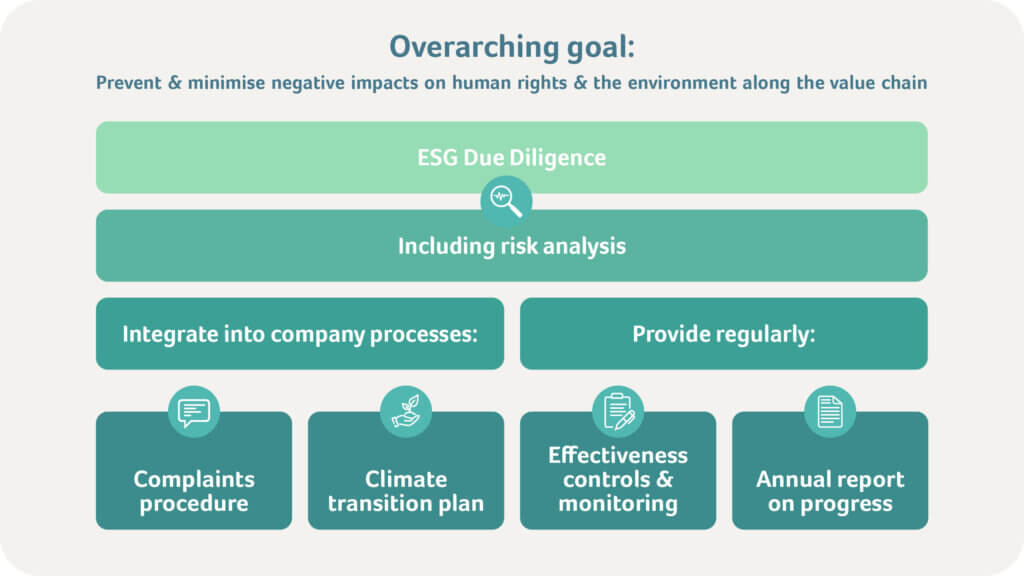
Comprehensive due diligence obligations that encourage companies to identify, mitigate, and address risks are at the core of the CSDDD. The key requirements include:
Risk Assessment & Analysis
Companies must identify potential and actual adverse impacts of their business operations and supply chains on human rights and the environment.
Prevention & Mitigation Measures
Appropriate measures must be implemented to minimise or prevent identified risks. This includes collaborating with suppliers and adapting internal processes.
Remediation & Grievance Mechanisms
A complaint mechanism must be established across the entire value chain and made accessible to all stakeholders. In cases of violations, companies must take measures to compensate those affected or improve the situation.
Climate Transition Plan
A Climate Transition Plan (CTP) must be implemented to address climate protection goals.
Transparency & Reporting
Companies are required to regularly report on their due diligence processes and outcomes aligning with the Corporate Sustainability Reporting Directive (CSRD) and European Sustainability Reporting Standards (ESRS), to enhance transparency and allow stakeholders to evaluate corporate responsibility.
What are the implementation timings for the CSDDD?
The EU has adopted a phased approach to implementing the CSDDD. Member States are required to transpose the directive into national law by 26 July 2026, and companies must comply with their obligations based on their size and revenue:

How will the CSDDD impact the medical device industry?
Within the scope of the CSDDD, Swiss manufacturers face several challenges as they navigate compliance.
Resource intensity
The directive mandates detailed due diligence systems for supply chains, encompassing supplier screening for environmental and human rights risks, grievance mechanisms accessible to stakeholders, and effectiveness monitoring.
For the medical devices industry, with its complex, multi-tiered supply chains, implementing these measures will require substantial resources.
Identifying risks along complex global supply chains can be resource-intensive and often requires substantial investment in technology and personnel. To meet the requirements under CSDDD, manufacturers may need to shift to suppliers with proven environmental, social, and governance (ESG) credentials or bring critical processes in-house.
Increased administrative burden
Reporting and documentation obligations add to the administrative burden, as companies must align with the Corporate Sustainability Reporting Directive (CSRD) and provide annual updates on progress in preventing human rights violations and environmental harm.
Supply chain management complexity
Whilst the directive builds on existing regulations such as the MDR and ISO 13485 standards for quality management, compliance with CSDDD requirements extends beyond product quality and safety to encompass ESG factors across supply chains.
The complexity of supply chains in the medical devices industry exacerbates this challenge. Manufacturers often rely on multi-tiered suppliers for raw materials, precision components, and specialised technologies, and ensuring compliance with both direct and indirect suppliers, particularly those without direct contractual relationships, requires robust systems and processes. Visibility of a supplier’s upstream practices, especially in regions with less stringent regulations, also remains a persistent challenge.
Medical device companies will need to adapt their procurement practices to align with CSDDD criteria, which could potentially mean the restructuring of their supply chains.
Cost implications
Compliance with the CSDDD will impose significant costs for manufacturers. Investments in risk-based ESG systems, training programs, and supplier audits are necessary to meet the directive’s requirements. These expenses may strain small / mid-sized manufacturers, as even though this size of company is not directly included within the scope of the directive, they are indirectly affected as part of the supply chains of larger companies, facing additional financial and administrative pressures.
What opportunities does the CSDDD present for medical device manufacturers?
Despite these challenges, compliance with the CSDDD directive may serve as a competitive differentiator in a market where ethical practices are increasingly valued. The CSDDD offers opportunities for Swiss manufacturers to proactively align with sustainability requirements – which may serve to enhance their corporate reputation and build customer trust, facilitate supply chain efficiency, and encourage innovation and collaboration.
Enhanced corporate reputation
Many Swiss companies have already adopted ESG frameworks. The directive formalises and expands these initiatives, enabling businesses to meet EU standards and improve their reputation for sustainability.
Supply chain efficiency
The standardised framework introduced by the CSDDD streamlines supplier requirements, reducing inconsistencies and redundancies for SMEs supplying EU-based manufacturers. This creates efficiencies that benefit the entire supply chain.
Encouragement of innovation & collaboration
The directive prompts companies to rethink their supply chains, which encourages innovation. For the medical devices industry, this could lead to the increased adoption of sustainable materials and advancement in product lifecycle management.
On a broader scale, the medical device industry may see increased collaboration as companies work together to standardise supplier requirements and reduce compliance costs, and industry associations facilitate collective ESG auditing and reporting.
What action should Swiss medical device manufacturers take?
To be prepared and proactive, Swiss manufacturers should start with comprehensive risk assessments to map their entire supply chain and identify high-risk areas, with priority given to regions or suppliers associated with human rights violations or environmental harm. Investing in technologies like digital supply chain traceability platforms and blockchain can improve transparency and accountability.
Engaging with suppliers is critical. Providing training to help them meet CSDDD requirements and developing long-term partnerships with aligned suppliers are essential steps.
Manufacturers should align internal reporting mechanisms with CSRD and CSDDD standards, leveraging pre-existing sustainability initiatives like Climate Transition Plans to streamline efforts.
Finally, collaboration within the industry is crucial. We would encourage manufacturers to join industry forums or alliances focused on CSDDD implementation to share best practices and pool resources for supplier audits. Advocacy with policy makers and industry groups also remains important to ensure the Swiss medical device sector’s concerns are represented in EU discussions.
In conclusion…
Although the CSDDD is a European initiative, it has far-reaching implications for the global economy, marking a crucial step toward sustainable and responsible corporate governance. Non-EU companies operating in Europe must also comply, encouraging alignment of international standards. As such, the directive sets an important benchmark for countries and regions outside of the EU to introduce stricter sustainability regulations.
By calling on companies to adapt their strategies and place sustainable values at the heart of their business practices, the CSDDD has the potential to drive positive global change. While this requires investment and commitment, it offers long-term business benefits like increased competitiveness and stronger stakeholder trust, and broader societal and environmental benefits like better working conditions and preservation of natural resources.
For Swiss medical device manufacturers, compliance with the CSDDD will demand significant financial investment and restructuring. But it also provides an opportunity to secure access to the EU market and align with global expectations. By proactively adopting the directive’s principles, Swiss manufacturers can mitigate risks, drive innovation, and position themselves as frontrunners in sustainable medical device development.
Our MedTech experts are ready to help with your pressing questions or challenges related to the CSDDD and medical devices – simply get in touch to start the conversation.
And stay tuned for the upcoming part three of our EU Green Deal Series covering the Ecodesign for Sustainable Products Regulation (ESPR).

