As health care advances, software has become integrated widely into digital ecosystems that serve both medical and non-medical purposes. Our Head of eHealth Paul Gardner shares advice on how to determine if your software product should be treated as Software as a Medical Device in our topical article below.
There are three types of software utilised within medical devices:
- Software as a Medical Device (SaMD): Software which is a medical device
- Software in a medical device: Software which is integrated into a medical device
- Software used in the manufacture or maintenance of a medical device
The following information focusses on type 1, determining whether your software is SaMD.
What is SaMD?
The International Medical Device Regulators Forum (IMDRF) defines Software as a Medical Device as:
“software intended to be used for one or more medical purposes that perform these purposes without being part of a hardware medical device.”
In other words, think of SaMD as software which is a medical device on its own. For instance, the medical software used to view images from diagnostic equipment on your phone would be SaMD. But the software that enables the diagnostic to run its test would not be. To be considered SaMD, software must not principally drive a hardware device.
Is my software a Medical Device?
To determine if your software is a medical device there are several helpful resources available.
For the EU market, the MDCG, which is composed of representatives from all Member States of the EU and chaired by a representative of the European Commission, provides guidance on qualification and classification of software within MDCG 2019-11.
For the US market, the FDA provides guidance on how to determine if your product is a medical device here.
How do I determine if my software meets the definition of a medical device?
MDCG 2019-11 and Section 201(h) of the US Food Drug and Cosmetic Act both contain clear definitions of a medical device.
Per Section 201(h) of the Food, Drug, and Cosmetic Act, a medical device is:
“An instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part, or accessory which is:
- recognized in the official National Formulary, or the United States Pharmacopoeia, or any supplement to them,
- intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, or
- intended to affect the structure or any function of the body of man or other animals, and
which does not achieve its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of its primary intended purposes.”
The term “device” does not include software functions excluded pursuant to section 520(o).
Per MDCG 2019-11, “medical device” means:
“Any instrument, apparatus, appliance, software, implant, reagent, material, or other article intended by the manufacturer to be used, alone or in combination, for human beings for one or more of the following specific medical purposes:
- diagnosis, prevention, monitoring, prediction, prognosis, treatment, or alleviation of disease,
- diagnosis, monitoring, treatment, alleviation of, or compensation for, an injury or disability,
- investigation, replacement, or modification of the anatomy or of a physiological or pathological process or state,
- providing information by means of in vitro examination of specimens derived from the human body, including organ, blood, and tissue donations,
and which does not achieve its principal intended action by pharmacological, immunological, or metabolic means, in or on the human body, but which may be assisted in its function by such means.”
To determine if your product meets the definition of a medical device, you first need to define the intended use and indications for use of your product. If you are uncertain in your assessment then I recommend referring to the FDA guidance here, or follow the simple decision steps outlined by the flowchart below, taken from the EU Commission’s resource: Is your Software a Medical Device?
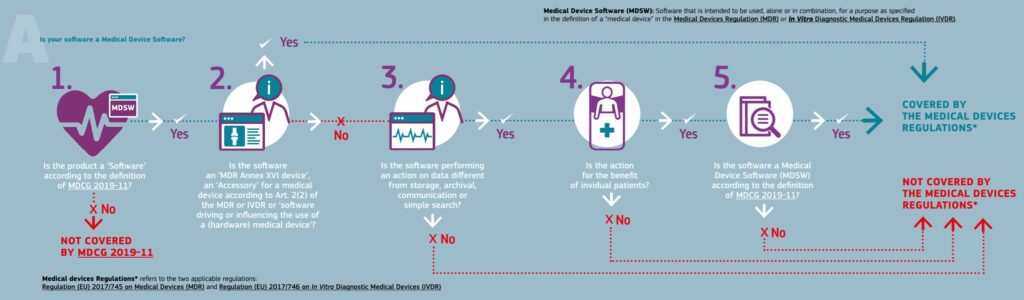
My software does not fall under the definition of a medical device. What should I do?
Should you determine that your software does not come under the definition of a medical device, then it is recommended that you follow best practice and develop the software following a QMS designed to meet the regulations, as you may change the intended use at some future point such that it does then meet the definition of a medical device.
I have determined my software is a medical device. What next?
Having determined that your software potentially is a medical device then additional considerations apply for the US market. If your software is a Mobile Medical Application, then the FDA has indicated that it intends to focus only on those device software functions that present a greater risk and you may benefit from their intention to exercise enforcement discretion as detailed in their Policy for Device Software Functions and Mobile Medical Applications.
With your software defined as a medical device, the next step is to then determine the device classification.
We will soon be sharing a follow up article from Paul on how to determine the device classification of your SaMD. In the meantime, our fact sheets on classifying medical devices more generally are available here for the EU and here for the US.


